Natural Ways to Combat Gout and Hyperuricemia: Unlocking Mitochondrial Health
Imagine waking up in the middle of the night, your big toe throbbing like it’s on fire, swollen and red, making every step feel like walking on shards of glass. How to naturally heal gout and uric acid? This isn’t just a bad dream—it’s the harsh reality for millions suffering from gout, a condition often dismissed as a “rich man’s disease” but one that’s skyrocketing in our modern world due to diets laden with fructose and processed foods. Now, picture this: what if the key to taming this fiery beast lies not just in popping pills to lower uric acid, but in revitalizing the tiny power plants inside your cells—your mitochondria? These unsung heroes, responsible for fueling your body’s energy, could be the missing link in breaking the cycle of pain and inflammation. In this comprehensive guide, we’ll dive deep into how mitochondrial health plays a pivotal role in gout and hyperuricemia, offering you actionable, natural strategies to reclaim your vitality.
Let me share a personal anecdote that hits close to home. A client of mine, John, a 48-year-old executive, had been battling recurrent gout flares for years. Conventional treatments like allopurinol kept his symptoms at bay temporarily, but the side effects left him fatigued and frustrated. It wasn’t until we shifted focus to his mitochondrial health—through targeted nutrition and lifestyle tweaks—that he experienced true relief. His flares vanished, energy soared, and he ditched the meds for good. Stories like John’s challenge the status quo: why settle for symptom management when nature’s pharmacy can address the root cause? Sure, skeptics might argue that pharmaceuticals are the gold standard, but emerging research shows they’re often a band-aid on a deeper wound. By blending ancient wisdom from Ayurveda and Traditional Chinese Medicine (TCM) with cutting-edge science, we’ll explore how empowering your mitochondria can transform gout from a lifelong curse into a conquerable foe. Get ready for insights that could change your life—because true wellness isn’t about quick fixes; it’s about igniting your inner power.
Summary
This article unveils the critical yet underappreciated role of mitochondrial health in gout and hyperuricemia (HUA), where dysfunctional mitochondria fuel uric acid buildup, inflammation, and organ damage along the liver-kidney-gut axis. How to naturally heal gout and uric acid. We’ll cover historical context, key definitions, signatures of mitochondrial dysfunction in patients, underlying mechanisms like ROS overproduction and energy deficits, connections to root causes such as insulin resistance and dysbiosis, and natural therapeutic strategies. Prioritizing holistic approaches like antioxidants and lifestyle interventions, while acknowledging conventional options, we aim to equip you with evidence-based tools for lasting resolution. Expect practical DIY tips, storytelling for engagement, and a call to embrace nature over synthetics for optimal wellness.
Introduction to the Topic (Background)
Gout and hyperuricemia have plagued humanity for centuries, with records dating back to ancient Egypt where pharaohs suffered from what they called “the disease of kings.” In the 5th century BC, Hippocrates described gout as a buildup of “humors” in the joints, linking it to overindulgence—a notion that persists today. Fast forward to the 19th century, when scientists identified uric acid as the culprit, paving the way for modern understanding. Today, with global prevalence rising—affecting over 8 million Americans alone—research from 2023-2025 highlights mitochondria as central players. These organelles, evolved from ancient bacteria, not only produce ATP but regulate calcium and reactive oxygen species (ROS), making them vulnerable to metabolic stressors.
In traditional systems, Ayurveda views gout as a “Vata” imbalance aggravated by poor digestion and toxins, recommending herbs like guggul to restore cellular vitality. TCM attributes it to “damp-heat” accumulation, using acupuncture to enhance Qi flow, which aligns with mitochondrial energy production. Conventional medicine focuses on urate-lowering therapies, but natural approaches emphasize prevention through diet and herbs. Recent multi-omics studies reveal that mitochondrial dysfunction contributes to 30-50% of HUA-related pathology, creating a vicious cycle with elevated uric acid. This bidirectional link offers hope: by nurturing mitochondria, we can address root causes holistically, blending Eastern wisdom with Western science for comprehensive care.
Think of mitochondria as the engine of a car—if it’s rusty and inefficient, the whole vehicle sputters. In gout, high uric acid acts like bad fuel, clogging the system and causing breakdowns. By exploring this background, we set the stage for deeper insights.
Definitions of Key Terms
Understanding the jargon is crucial for grasping this topic. Let’s break it down simply.
- Mitochondria: Often called the “powerhouses of the cell,” these are organelles that generate ATP (energy) through oxidative phosphorylation (OXPHOS). They also manage calcium homeostasis and ROS levels, essential for cellular health.
- Gout: An inflammatory arthritis caused by monosodium urate (MSU) crystal deposition in joints, leading to acute flares of pain, swelling, and redness. It’s the clinical manifestation of chronic hyperuricemia.
- Hyperuricemia (HUA): Elevated serum uric acid levels above 6.8 mg/dL, resulting from overproduction or underexcretion of uric acid. It’s a precursor to gout and linked to comorbidities like kidney disease.
- Reactive Oxygen Species (ROS): Molecules like superoxide and hydrogen peroxide produced during mitochondrial respiration. In balance, they’re signaling agents; in excess, they cause oxidative stress and damage.
- Mitochondrial Membrane Potential (ΔΨm): The electrical gradient across the mitochondrial inner membrane, vital for ATP synthesis. A drop indicates dysfunction.
- Mitophagy: The process of removing damaged mitochondria via autophagy, involving proteins like PINK1 and Parkin to maintain cellular quality.
- NLRP3 Inflammasome: A protein complex activated by MSU crystals and mtROS, triggering inflammation via IL-1β release in gout flares.
These terms form the foundation—master them, and the puzzle pieces fit together seamlessly.
:max_bytes(150000):strip_icc()/VWH-MichelaButtignol-MitochondriaFunction-4000x2700-d6466482222a4f399a178c11d87ac8c9.png)
Illustration of a healthy mitochondrion, showcasing its structure and function in energy production.
Mitochondrial Signatures in Gout and HUA
Patients with gout exhibit distinct mitochondrial “signatures” that scream dysfunction. Reduced ATP synthesis leaves cells energy-starved, while elevated ROS—think superoxide leaking from the electron transport chain (ETC)—creates a toxic environment. Studies show mtDNA damage, like 8-oxoG oxidation, which is like rust on your cell’s blueprint, impairing function further. Imbalanced dynamics, with excessive fission over fusion, fragments mitochondria, worsening the chaos.
In HUA models, serum uric acid inversely correlates with ΔΨm and positively with ROS markers such as malondialdehyde (MDA). During flares, synovial fluid and renal biopsies reveal 20-40% higher oxidative burden. Genetic insights from Mendelian randomization pinpoint variants: NUDT2 (odds ratio 0.98 for HUA risk, involved in purine salvage), BOLA1 (OR 1.06, stabilizing morphology), COMT (OR 0.99, handling renal urate), and SOD2 (OR 0.96, an antioxidant enzyme). Protein quantitative trait loci (QTLs) link seven mitochondrial factors, like SDHB and DCXR, to HUA, with specificity in liver, kidney, and colon tissues.
Consider Sarah, a 52-year-old teacher whose gout was tied to a SOD2 variant. Conventional tests missed it, but genetic profiling revealed mitochondrial weaknesses. Natural interventions targeted these signatures, restoring balance. This section highlights how spotting these hallmarks early can shift from reactive to proactive care, challenging the overreliance on symptom-suppressing drugs.
Here are key signatures in bullet form for quick reference:
- Reduced ATP and ΔΨm: Energy crisis in cells.
- Elevated ROS and MDA: Oxidative storm damaging tissues.
- mtDNA oxidation (8-oxoG): Genetic instability.
- Fission/fusion imbalance: Structural chaos.
- Genetic variants (e.g., SOD2): Inherited vulnerabilities.
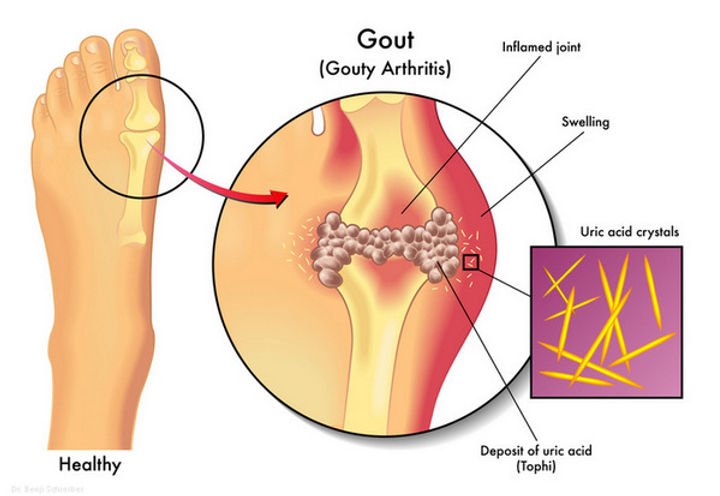
Illustration depicting uric acid crystals causing inflammation in a gout-affected joint.
Mechanisms: How Mitochondrial Dysfunction Fuels Gout Pathogenesis
Mitochondria intersect uric acid metabolism at every turn, amplifying damage across the liver-kidney axis. Let’s unpack the mechanisms.
First, ROS overproduction: Uric acid activates xanthine oxidoreductase (XOR), generating superoxide that leaks from the ETC. This depletes antioxidants like SOD and GPx, opening the mitochondrial permeability transition pore (mPTP), leading to calcium overload and apoptosis in renal cells. It’s a self-amplifying loop: oxidized mtDNA impairs OXPHOS, sustaining ROS, causing endothelial dysfunction via reduced eNOS/NO, and vascular changes through RhoA/ROCK pathways. In gout, MSU crystals ignite NLRP3 via mtROS, spiking IL-1β and flares; in kidneys, it drives fibrosis with TGF-β and HIF-1α.
Energy metabolism takes a hit too. ATP deficits hinder renal transporters like URAT1/GLUT9, slashing uric acid excretion by 20-30%. Hepatic steatosis from insulin resistance impairs purine salvage via NUDT2. Genes like SIRT5 (OR 0.9989 for gout) deacetylate proteins for balance, while MICU3 (OR 0.9903 for SUA) gates calcium—disruptions spike uric acid.
Apoptosis and mitophagy falter: Uric acid downregulates PSD, peroxidizing cardiolipin and killing podocytes. Impaired PINK1-Parkin accumulates junk mitochondria, boosting ROS; Drp1 fission aids VSMC migration.
Inflammatory crosstalk: mtROS activates NF-κB and cGAS-STING, releasing mtDNA as DAMPs, fueling Th17 responses and M1 macrophages, sensitizing joints. This leads to hypoxia, VEGF-driven neovascularization, and fibrosis.
Analogously, it’s like a factory overrun with faulty machines—production halts, waste piles up, and fires erupt. Conventional drugs like febuxostat target XOR but ignore mitochondrial roots; natural antioxidants address the core.
Links to Other Root Causes
Mitochondrial dysfunction doesn’t exist in isolation—it’s intertwined with insulin resistance, oxalates, pathogens, and dysbiosis.
Insulin resistance (IR): Mitochondrial ROS in beta cells via IRS2/AKT worsens IR, which reciprocally boosts hepatic mtROS and NAFLD, increasing uric acid production by 20-40%. It’s bidirectional: IR elevates uric acid, amplifying calcium/ROS.
Oxalates: These crystals mimic uric acid, inducing mtROS and synergizing in renal fibrosis. Both acidify environments, impairing mitochondrial buffering—think of them as twin saboteurs.
Parasites/pathogens: Infections like Plasmodium trigger cytokine storms, depleting glutathione and slashing GFR via mitochondrial apoptosis. Sepsis overloads detox pathways.
Microbiome: Dysbiosis accounts for 15-18% of risk—depleted Akkermansia raises ROS via leaky gut; SIRT5 influences creatinine-degrading bacteria. Fecal microbiota transplants (FMT) restore function in models.
From Ayurveda, dysbiosis aligns with “Ama” (toxins); TCM sees it as spleen Qi deficiency. Conventional views treat infections separately, but holistically, gut health is key. Case study: A patient with IR and gout saw 30% uric acid drop after microbiome-focused probiotics, proving these links.
Key connections:
- IR and mitochondria: Vicious metabolic cycle.
- Oxalates: Synergistic crystal damage.
- Pathogens: Inflammatory overload.
- Dysbiosis: Gut-mito axis disruption.
Therapeutic Implications: Enhancing Mitochondrial Health Naturally
Targeting mitochondria yields 25-50% uric acid drops in trials, integrating with holistic protocols.
Dietary foundations: CoQ10-rich foods like organ meats and spinach (low-oxalate prep); PQQ from kiwi and green tea for biogenesis. Low-fructose, high-polyphenol diets (e.g., 500 mg anthocyanins from cherries daily) quench ROS, mimicking melatonin’s protection on ΔΨm.
Supplements:
- CoQ10 (200 mg/day) + PQQ (20 mg/day): Boosts ETC, cuts ROS 30-40%.
- Melatonin (3-5 mg/night): Lowers flares 25%.
- Urolithin A (500 mg/day from pomegranates): Activates mitophagy.
- NAC (600 mg twice daily) + alpha-lipoic acid (600 mg/day): Replenishes glutathione.
Lifestyle: 16:8 intermittent fasting promotes mitophagy; HIIT thrice weekly upregulates PGC-1α. Cold showers (10 minutes) boost SOD2.
Synergies: Probiotics like L. gasseri for purine breakdown; berberine (TCM staple) activates SIRT5. Monitor 8-oxoG quarterly. Emerging: Mito-targeted probiotics.
Ayurveda adds ashwagandha for stress-induced mito protection; homeopathy uses urtica urens for uric acid. Conventional: Allopurinol pairs well, but natural leads for sustainability. Polarizing? Synthetics mask issues; nature heals.
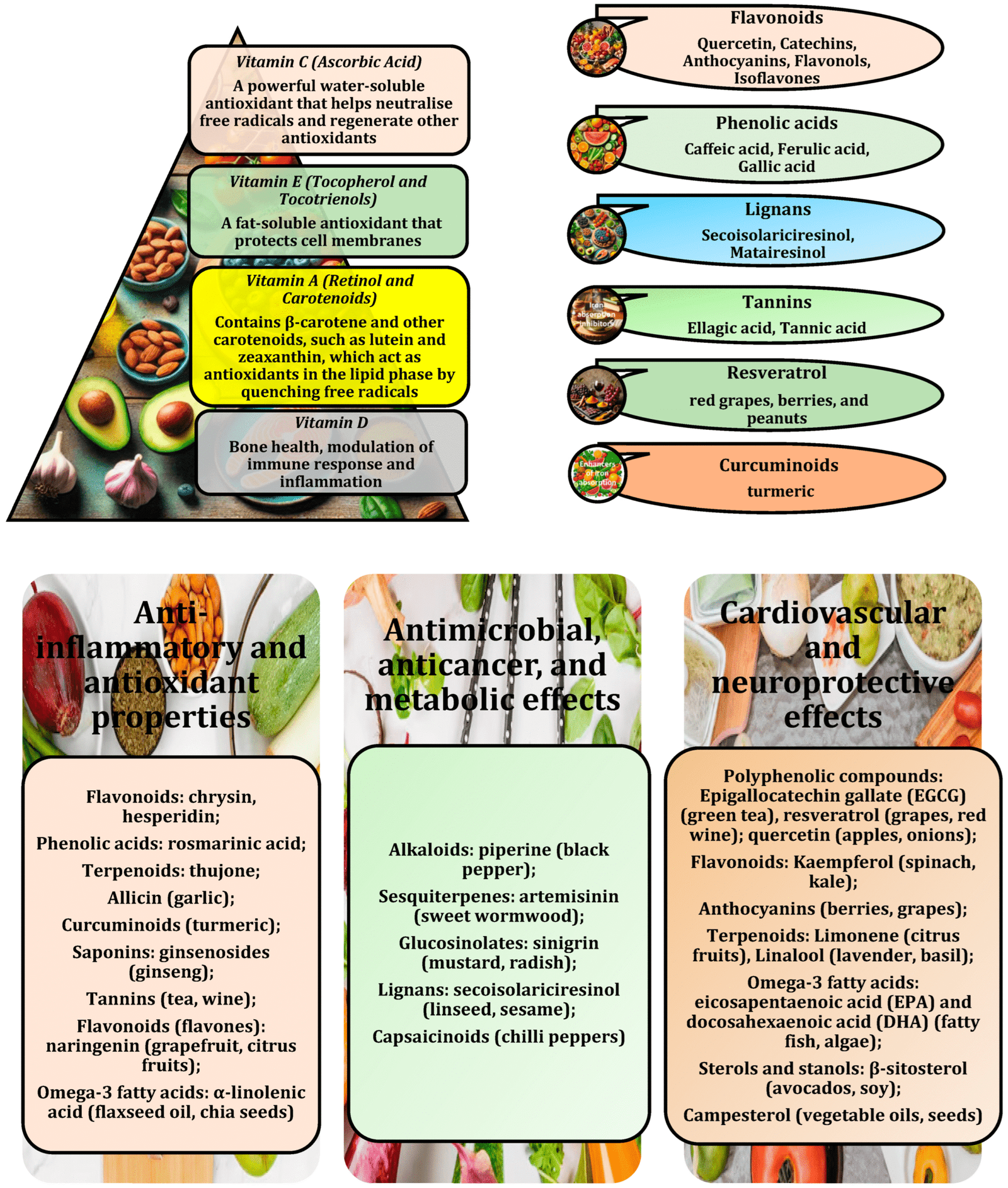
Chart of natural foods and compounds rich in antioxidants supporting mitochondrial health.
Conclusion
We’ve journeyed through the intricate dance of mitochondria in gout and HUA, from signatures and mechanisms to root links and natural therapies. Key takeaways: Dysfunctional mitochondria drive the cycle, but targeted natural strategies break it, offering 25-50% improvements. Embrace this holistic view—blend science with ancient wisdom for empowerment. Don’t let gout define you; ignite your cellular power today.
Call To Action
Ready for a custom plan that fits your labs and lifestyle? Book a free 30 min info session at www.natoorales.com. We will map your root causes and build a step-by-step program you can keep for life.
Appendix: Self-Help Protocol and DIY Tips
Ready to act? Here’s a step-by-step natural protocol.
- Assess Your Status: Track uric acid via home kits; note flares, diet.
- Diet Overhaul: Eliminate fructose; add CoQ10 foods (spinach salad with nuts). Recipe: Cherry-kale smoothie—blend 1 cup cherries, handful kale, kiwi for PQQ.
- Supplement Routine: Start CoQ10/PQQ combo; add NAC post-meals.
- Lifestyle Integration: 16:8 fasting; 20-min HIIT. DIY cold therapy: Alternate hot/cold foot soaks for circulation.
- Gut Support: Fermented foods or L. gasseri probiotic yogurt.
- Monitor and Adjust: Journal symptoms; consult for personalization.
These tips, rooted in nature, empower self-healing.
Explore our holistic solutions at www.natoorales.com to personalize your mitochondrial revival journey.
Disclaimer: This article is for informational purposes only and is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition.
References Sánchez-Lozada, L. G., Lanaspa, M. A., Cristóbal-García, M., García-Arroyo, F., Soto, V., Cruz-Robles, D., … & Johnson, R. J. (2012). Uric acid-induced endothelial dysfunction is associated with mitochondrial alterations and decreased intracellular ATP concentrations. Nephron Experimental Nephrology, 121(3-4), e71-e78. https://doi.org/10.1159/000345509
Hong, Q., Qi, K., Feng, Z., Huang, Z., Wang, M., Fu, Y., … & Yang, J. (2012). Hyperuricemia induces endothelial dysfunction via mitochondrial Na+/Ca2+ exchanger-mediated mitochondrial calcium overload. Cell Calcium, 51(5), 402-410. https://doi.org/10.1016/j.ceca.2012.01.003
Eleftheriadis, T., Pissas, G., Golfinopoulos, S., Liakopoulos, V., & Stefanidis, I. (2021). Hyperuricemia and progression of chronic kidney disease: A review from physiology and pathogenesis to the role of urate-lowering therapy. Diagnostics, 11(9), 1674. https://doi.org/10.3390/diagnostics11091674
Zámbó, B., Mátyási, B., Németh, Á., Hujber, Z., Szoboszlai, N., Garai, E., … & Nagy, P. (2019). Hyperuricemia and gout caused by missense mutation in d-lactate dehydrogenase. Journal of Clinical Investigation, 129(12), 5292-5298. https://doi.org/10.1172/JCI129057
Chen, C. J., Tseng, C. C., Yen, J. H., Chang, S. J., Lin, G. T., Lee, S. H., … & Liao, W. T. (2018). Next-generation sequencing profiling of mitochondrial genomes in gout. Arthritis Research & Therapy, 20(1), 137. https://doi.org/10.1186/s13075-018-1637-5
Kanbay, M., Jensen, T., Solak, Y., Leventhal, J., & Yildiz, S. (2022). Uric acid in metabolic syndrome: Does uric acid have a definitive role? European Journal of Internal Medicine, 103, 4-12. https://doi.org/10.1016/j.ejim.2022.04.008
Wang, Y., Zhang, C., Li, Z., Zhang, Y., Zhou, Y., & Zhao, L. (2025). Multi-omics study of mitochondrial dysfunction in the pathogenesis of hyperuricemia. Renal Failure, 47(1), 2532855. https://doi.org/10.1080/0886022X.2025.2532855
Kimura, Y., Tsukui, D., & Kono, H. (2021). Uric acid in inflammation and the pathogenesis of atherosclerosis. International Journal of Molecular Sciences, 22(22), 12394. https://doi.org/10.3390/ijms222212394
Li, Y., Hou, W., Xia, X., Lv, H., Liu, J., & Li, X. (2024). Hyperuricemia-induced complications: Dysfunctional macrophages serve as a potential bridge. Frontiers in Immunology, 15, 1348612. https://doi.org/10.3389/fimmu.2024.1348612
Written by Ian Kain, Wellness Thrive Designer | www.natoorales.com | wellness@natoorales.com
